From Concept to Clearance
We Deliver Medical Devices
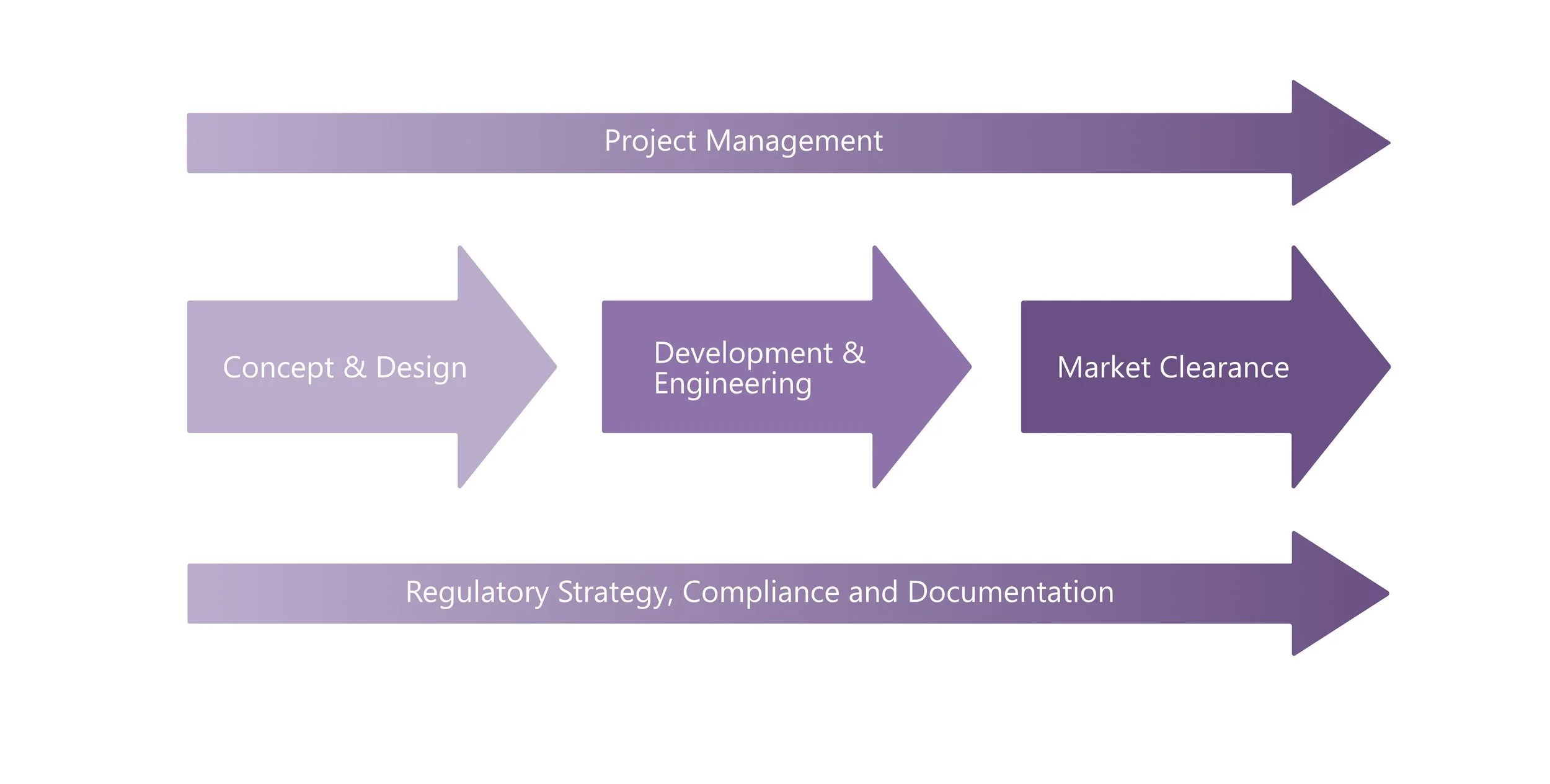
SechMed can manage your entire development project, or integrate seamlessly at key stages, from idea through design and on to market approval, guided by clear regulatory strategy, ensuring safe, effective and marketable devices
Realising Your Medical Device
Who We Are
SechMed is a product development partner specialising in medical devices, built on decades of collective experience in design engineering, regulatory affairs, and product realisation. We help innovators and companies transform ideas into safe & effective devices, ready for real-world use.
Our approach combines technical precision with regulatory insight, ensuring that projects stay on track. Whether delivering a full development program or strengthening a project at a critical stage, we provide the clarity, structure, and expertise needed to succeed.
Why Work with SechMed?
-
Full Product Realisation
SechMed goes beyond design alone. We manage the entire medical device development process, from concept through design, testing inclusive of usability, and manufacturing, with regulatory strategy integrated throughout. Our end-to-end approach ensures devices are delivered safely, effectively, and ready for market approval.
-
Specific Phase Support
Not every project needs a full development partner throughout. SechMed can integrate seamlessly at any phase of your device development cycle, providing specialist expertise where you need it most. Whether it’s regulatory input, design & usability engineering, or design validation, we adapt to your objectives without disrupting your project flow.
-
Trusted Partner
At SechMed, we don’t just deliver technical outputs; we align with your goals and act as a true development partner. Whether guiding a full programme or supporting at critical milestones, we take ownership of outcomes, communicate transparently, and stay invested in your success from start to finish
-
Experienced, Cohesive Team
Our team has worked together for over a decade, successfully developing medical devices across multiple surgical specialties. With decades of combined design experience and deep knowledge of the regulatory landscape, we bring proven expertise and a collaborative spirit that clients can trust to deliver results
What We Do
Design & Development
SechMed provides expert support across the early and pre-market phases of device development. We translate clinical needs into design requirements, generate concepts, and progress them into detailed CAD models and working prototypes. We build and iterate benchtop and pre-production models, undertake verification testing, risk analysis, and usability studies to guide device development and optimise performance. The result: devices that are technically robust, safe, and meet their intended use.
Regulatory & Compliance
Our regulatory expertise is applied practically, in parallel with development activities. Our Quality Management System is aligned to ISO 13485, FDA 21 CFR Part 820 and ISO 14971, ensuring that your product is in compliance at every stage. We can generate completed technical documentation to support FDA 510(k), De Novo, PMA, and EU MDR submissions. By ensuring compliance at every milestone, we avoid common bottlenecks at the final submission stage.
Manufacturing & Process Qualification
SechMed supports smooth transition from development to production. We assist with supplier selection, design transfer, process validation, and quality system integration. Our proactive approach to project planning ensures that the development of the manufacturing process keeps pace with the requirements of device testing.
Sechmed works to ensure a turnkey manufacturing process is provided to our clients.
How We Work
SechMed’s process is built to take devices through the entire development journey, from first ideas to market release and beyond. Our structured approach keeps projects moving forward with clarity, embedding compliance at every stage and ensuring that outcomes are safe, effective, and approval-ready.
SechMed can deliver the full development lifecycle, where our approach adds the most value, but our framework is also flexible enough to provide targeted support at critical stages, applying the same structured oversight and compliance focus to specific objectives within a broader programme.
The result is a development pathway that is clear and efficient.
Testimonials
Frank Bonadio, CEO at Advanced Surgical Concepts
“The team at SechMed have worked for many years developing a wide range of medical devices for Advanced Surgical Concepts (ASC). During this time, they brought over ten novel devices to market, from initial ideas through to market release by many of the world’s largest medical device companies, including Johnson and Johnson, Olympus, Bard/Davol and Covidian (Medtronic). The team has overseen sixteen 510k and two De Novo applications that were successfully cleared by FDA first-time. The SechMed team were instrumental in delivering these devices to market in designing novel solutions for complex surgical issues”
Contact Us
Interested in working together? Fill out some info and we will be in touch shortly. We can’t wait to hear from you!