How We Work
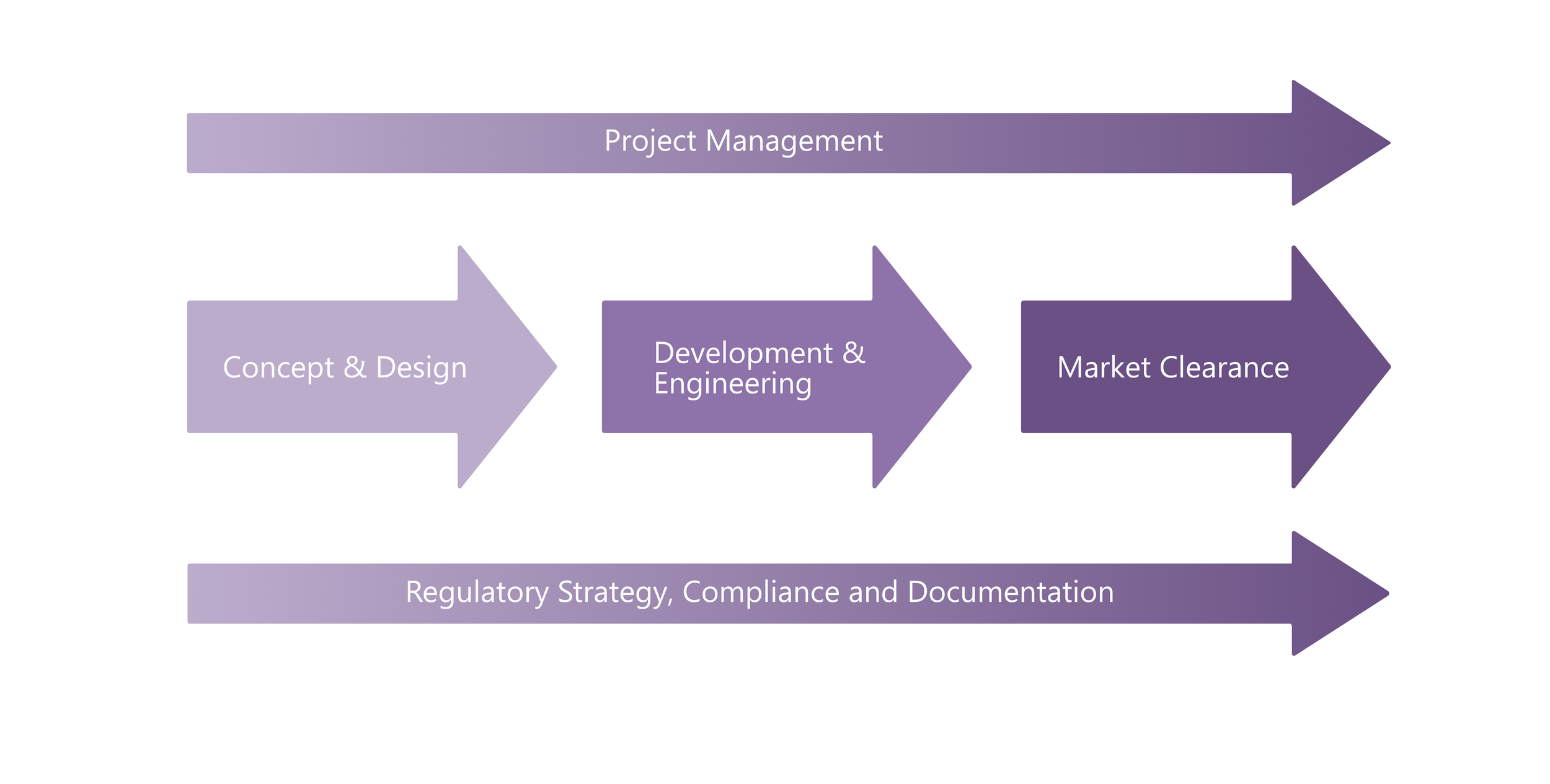
SechMed’s process is built to take devices through the entire development journey, from first ideas to regulatory approval and market release. Our structured approach keeps projects moving forward with clarity, embedding compliance at every stage and ensuring that outcomes are safe, effective, and approval-ready.
SechMed can deliver the full development lifecycle, where our approach adds the most value, but our framework is also flexible enough to provide targeted support at critical stages, applying the same structured oversight and compliance focus to specific objectives within a broader programme.
The result is a development pathway that is clear, efficient, and regulator-ready, whether managed end-to-end or strengthened at a key stage.
Project Management & Oversight
At the top level, we apply disciplined project management to align milestones, budgets, and risks. This creates clear decision points and full visibility, so that technical progress, regulatory needs, and commercial objectives stay tightly connected.
Concept & Design → Development & Engineering → Market Clearance
At the core of our process is the progression of your device itself. Early concepts are translated into structured designs, developed through prototyping and engineering, and refined into manufacturable products. From there, we prepare devices for production, validate processes, and manage the transition into manufacturing and market clearance.
Regulatory & Compliance in Parallel
Running alongside every stage, our regulatory expertise ensures that design history files, technical documentation, and risk management evolve in step with the device. By integrating regulatory strategy throughout, we remove bottlenecks and avoid the late-stage delays that can stall otherwise strong programmes.
Services We Offer
Technology Assessment
Device Design
Contract Manufacturer selection
Project Planning and Budgeting
Usability/Human Factors Testing (IEC 62366)
Risk Management (ISO 14971)
Develop device specifications and Drawings
Process development
Packaging Development and Testing
Tooling and Fixture Design
IFU development and translation
IFU development and translation
Process Validation
Sterilisation Validation
Design Verification
Design Validation: In Vivo or In Vitro testing
Biocompatability testing
Biocompatability Report
Design Transfer
Regulatory support; Pre-Submission meetings and Submission Documentation