Design & Development
SechMed provides expert support for medical device projects from early development through to market release. We translate clinical insights into clear design requirements, generate multiple innovative concepts, and progress them into detailed CAD models and functional prototypes. Our team iterates designs through benchtop and pre-production builds, undertaking verification testing, risk analysis, and usability studies to guide device development and optimize performance. Working to international standards to meet medical device regulations, we ensure every stage is documented, compliant, and ready to support regulatory submissions. The outcome is devices that are technically robust, and engineered to be intuitive for users
Feasibility
At the start of each project, SechMed investigates whether a concept is viable, both clinically and economically. This involves reviewing the existing market & competitor products, evaluating current technologies, and identifying relevant intellectual property. We also explore pricing expectations to determine target manufacturing costs & capacity and assess the regulatory pathways required for clearance. These early insights reduce downstream risk and guide projects toward commercially and clinically viable outcomes
User Needs Research
A successful medical device begins with a deep understanding of the user and the use environment. We collaborate with clinicians, patients, and stakeholders to capture real-world insights, identify unmet needs and use environment requirements. We translate these findings into measurable design inputs that anchor the entire development process. By engaging end users early, we reduce the risk of late-stage surprises and ensure the device is designed with practical application in mind.
Concept Generation
Our creative design process produces multiple unique solutions to each challenge, not just a single option. We explore form, function, and usability, presenting clear trade-offs and advantages for client review. Concepts are presented through sketches, 3D models, and rapid mock-ups. Encouraging early discussion with stakeholders helps establish & prioritise requirements and ensures the chosen design direction balances innovation, manufacturability, and user needs.
Prototyping & Testing
Prototypes are central to how we develop and refine devices. We use low-fidelity proof-of-principle prototypes, and high-fidelity models that closely match final form & function, to hone each design. We test each concept for usability, mechanical performance and reliability to guide the next design iteration. By testing early and often, we uncover potential risks and build confidence in the design before progressing to manufacturing
Human Factors Engineering/Usability
Usability/human factors engineering is integrated throughout our development process, meeting the requirements of IEC 62366. We perform task analysis, use-related risk assessments, and formative usability evaluations to ensure devices can be operated safely and effectively by the intended user population. By integrating human factors early, we not only meet regulatory requirements but also reduce the likelihood of user error, improving both safety and adoption in clinical practice. We conduct summative usability studies with representative end-users in an appropriate use environment.
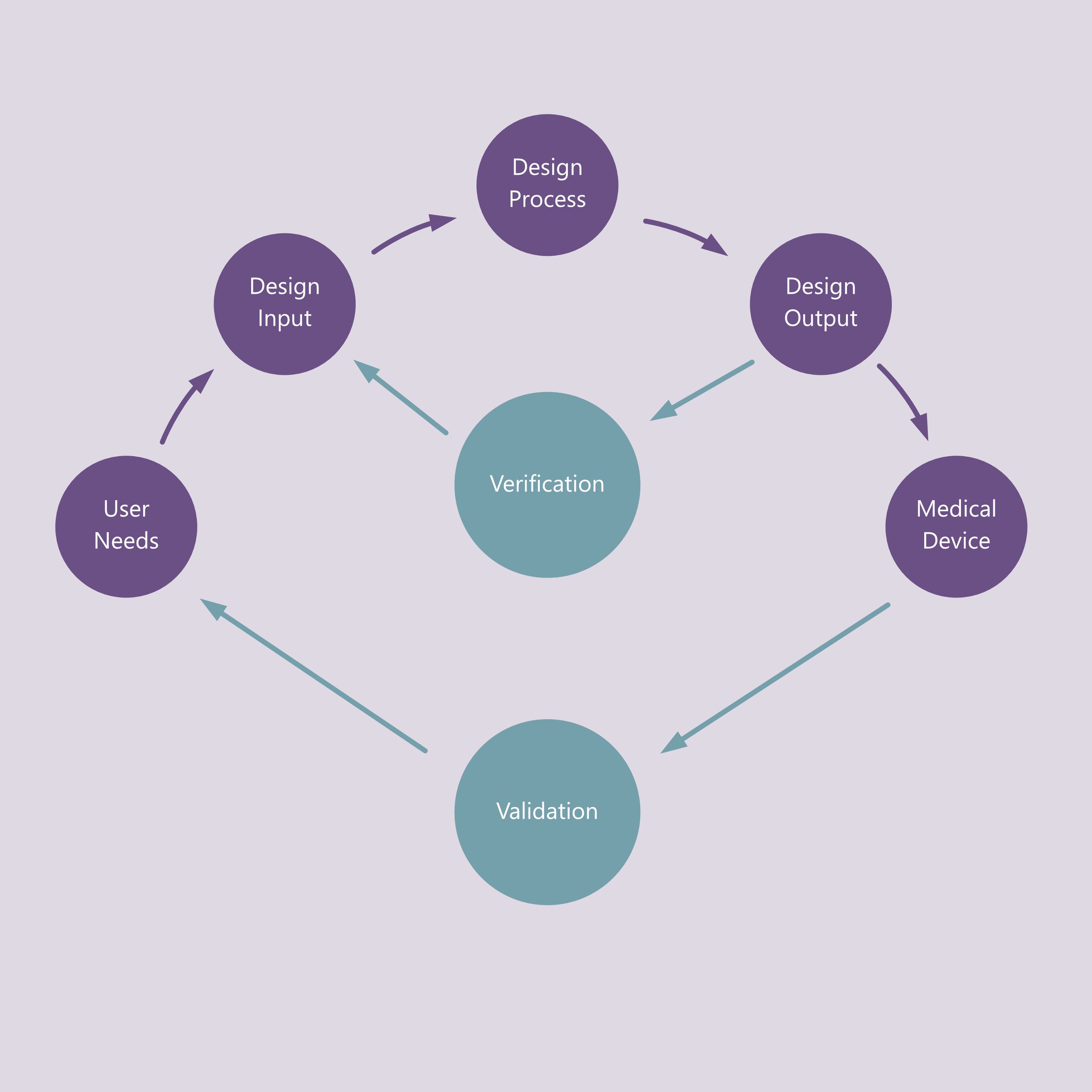
Design Verification and Validation
We operate within an ISO 13485–compliant quality framework, ensuring that every design activity is documented and traceable. Design inputs are clearly defined and are verified & validated to produce reliable and controlled design outputs. This structured approach generates robust design history files and technical documentation, streamlining the preparation of FDA 510k & De Novo, CE-mark applications, and other global submissions. Our clients benefit from both speed and confidence, knowing compliance is built in from day one.
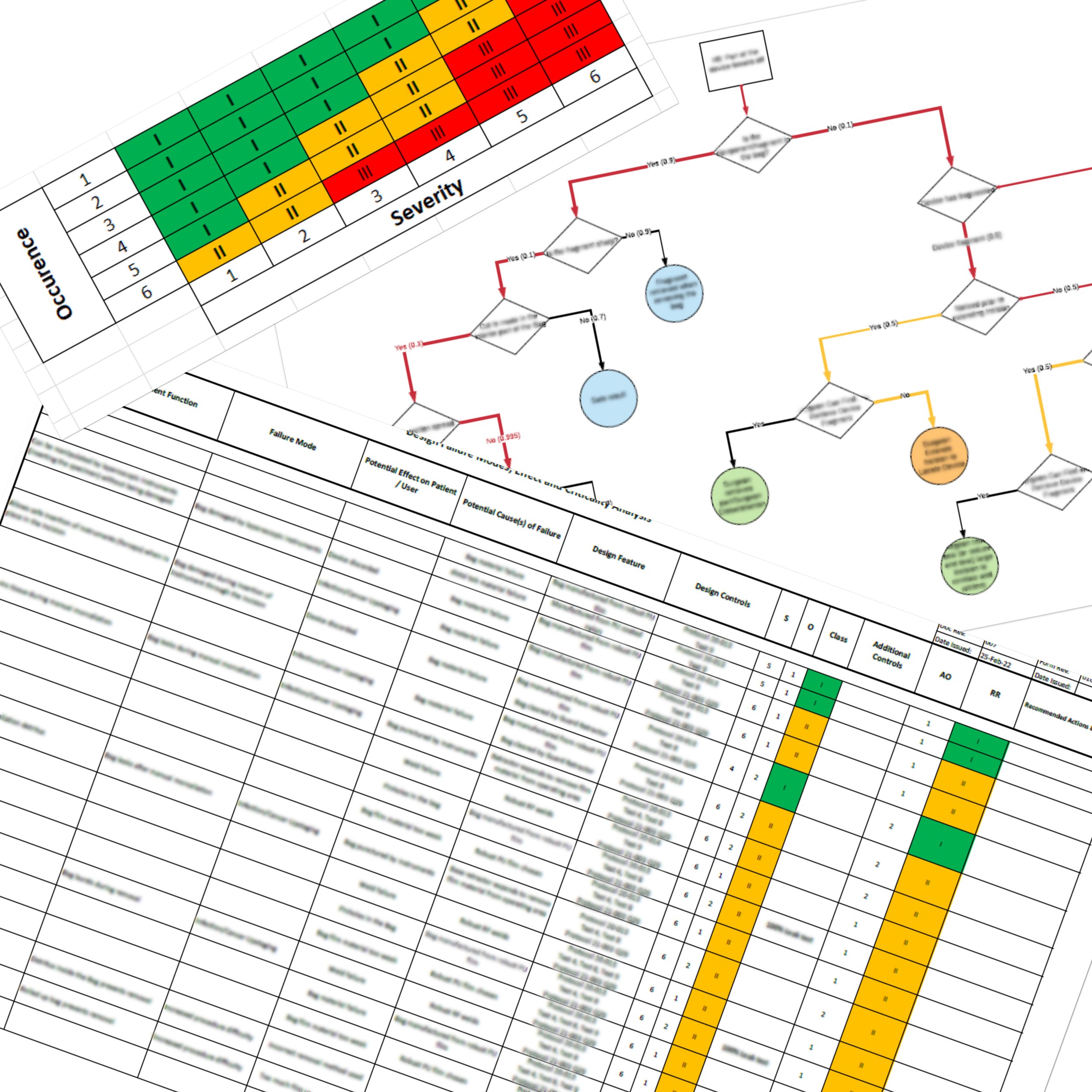
Risk Management
Risk management is integral to our development process. Following ISO 14971, we systematically identify hazards, estimate and evaluate risks, and implement effective mitigation strategies. Risk controls are verified and validated through testing, ensuring that safety requirements are consistently met. By treating risk management as a continuous process, rather than a late-stage exercise, we help clients avoid regulatory pitfalls and ensure safer outcomes for end users.
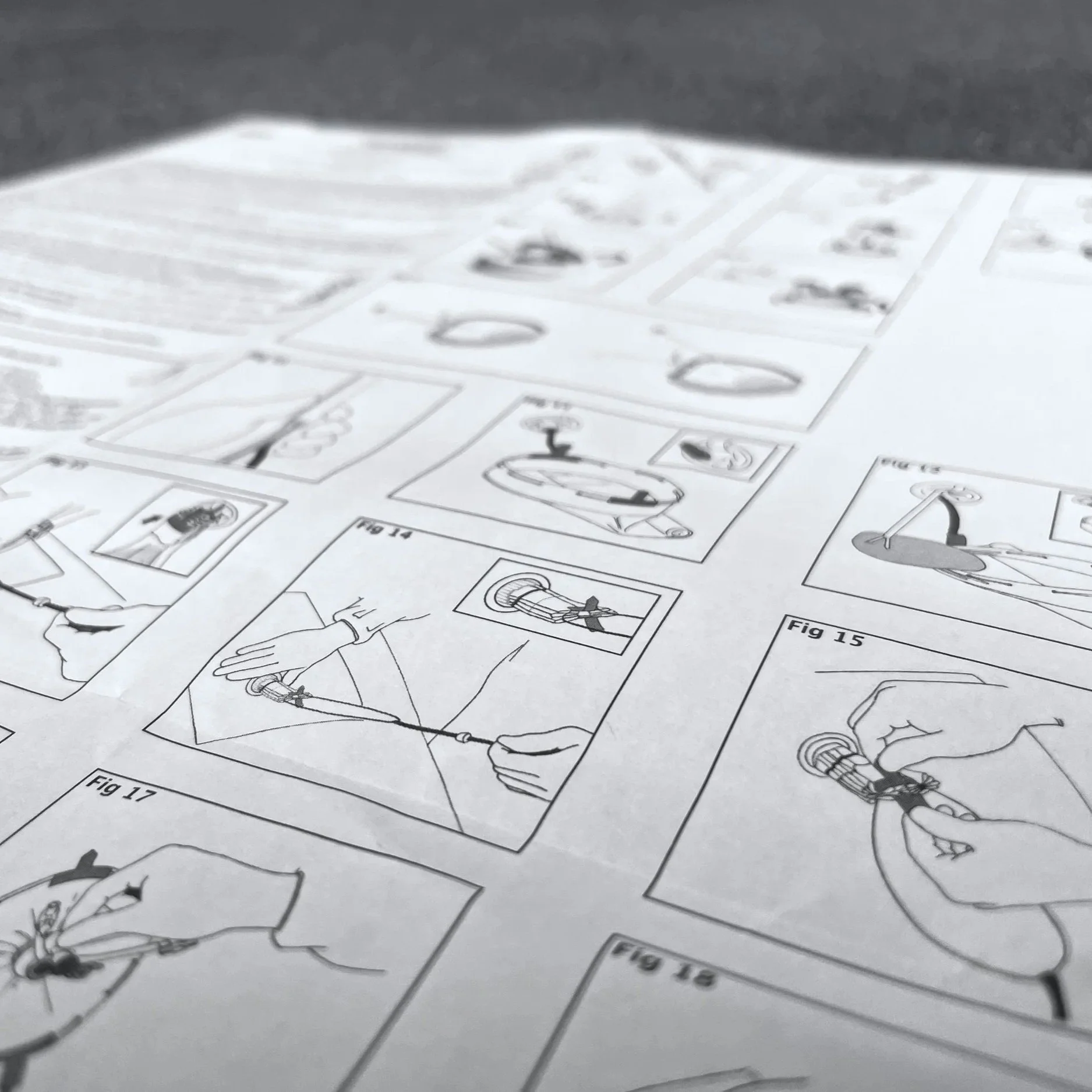
IFU, Labelling and Packaging
Clear, accurate labelling, Instructions for Use (IFUs), and packaging are critical for safe operation and traceability. SechMed develops IFUs and labelling in parallel with device design that meet regulatory requirements and labelling standards while remaining accessible and easy to understand. Packaging is designed and validated not only for protection and sterility, but also to support usability and intuitive product handling. These elements are incorporated into usability studies and validated to confirm clarity and effectiveness.
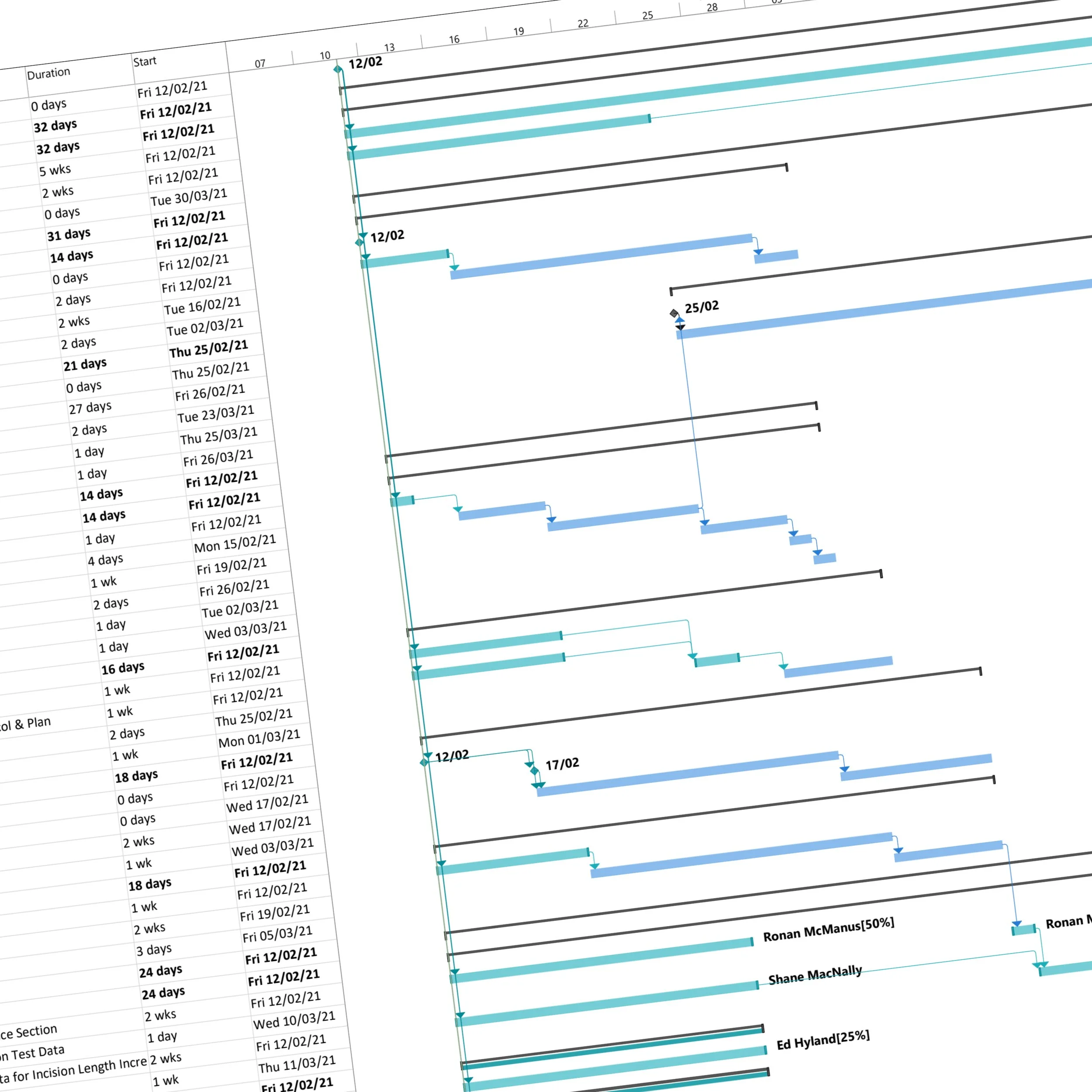
Project Management
Strong project management underpins everything we do. SechMed provides structured planning, milestone tracking, and budget control to keep projects on schedule and aligned with client goals. We incorporate regulatory strategy into project planning to reduce risk, minimise delays, and ensure that development decisions support eventual market clearance.